
Multiple Sclerosis

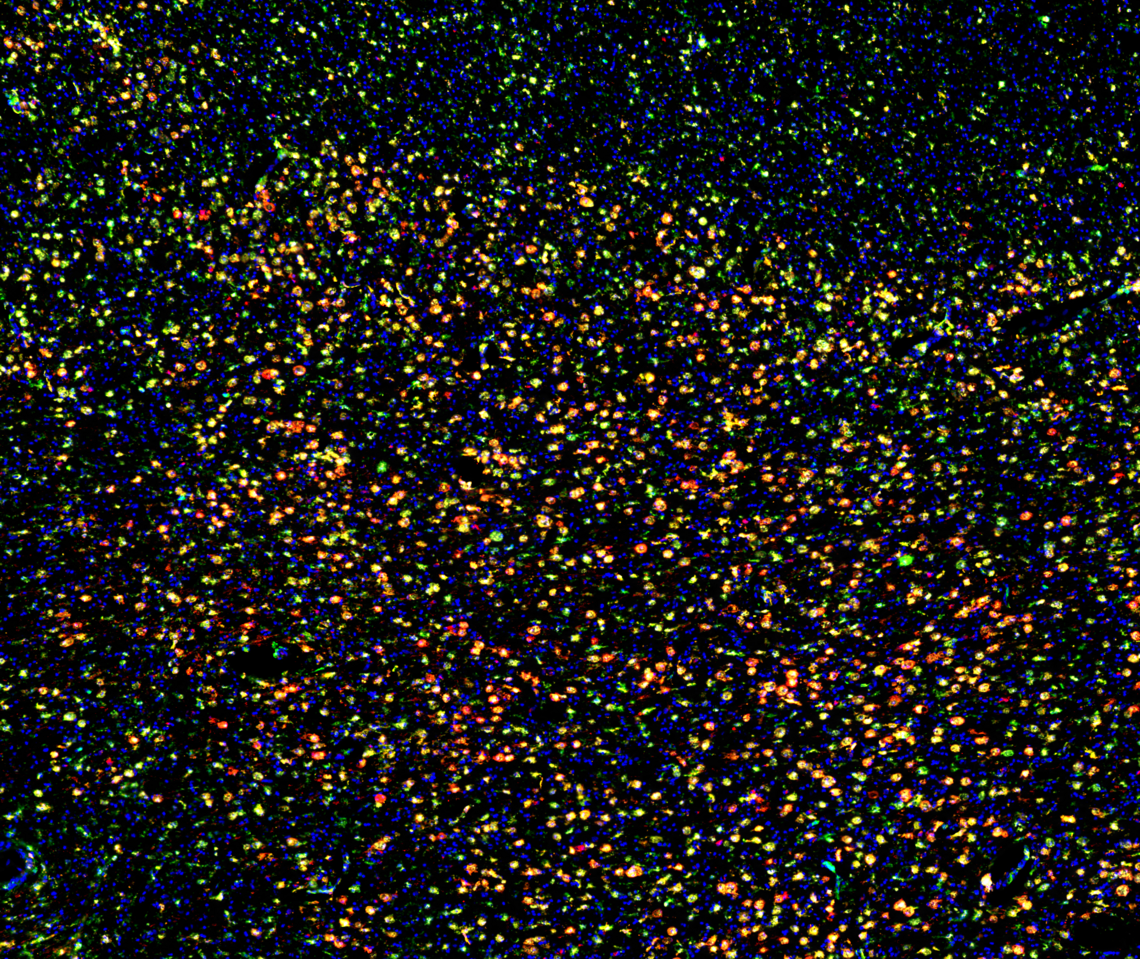
Plasma cells (CD138) localized in the subcapsular sinus (CD31) lined with macrophage (Iba1). B cell follicle filled with B cells (B220) that are primarily naive (IgD). T cell zone tilled with CD4+ T cell (CD4) and dendritic cells (MHC-II).
Rajiv Jain
Identifying pathogenic B cell subsets and their mechanisms in MS lesions
B cells can promote and suppress the progression of MS but we currently do not know which subsets of B cells exist in the brain, their locations in the brain, or the mechanisms that B cells are using to modulate MS progression. In my project, I will address these problems by fully characterizing the locations and subsets of B cells in MS lesions. Using an animal model and cells in culture, I will identify the major mechanism(s) that B cells are using to promote or suppress CNS damage and determine whether cellular interactions in MS lesions influence B cell pathogenesis.
Researcher: Rajiv Jain
Active MS lesion contains HLADR+ myeloid cells (red), many of which are NOX2+ (green). DAPI is used for nuclei (blue)
Atefeh Rayatpour
Neuroprotection and remyelination in MS by overcoming oxidative stress in lesions
Oxidative stress plays a major role in the pathogenesis of multiple sclerosis (MS). It is induced by generation of reactive oxygen and nitrogen species, mainly by activated microglia/macrophages in active MS lesions. Accumulating evidence suggests that oxidative damage of oligodendrocytes and axons occurs early in MS and continues unabated. Despite this, a comprehensive analysis of oxidative stress and protective pathways in MS and its animal models is lacking. Here, I’m performing deep analyses of RNA sequencing datasets and pathway analysis of various lesion types of MS and its animal models to illuminate a whole map of oxidative pathways and their mediators in lesions. Putative targets will be corroborated histologically, and then blocked by pharmacological and genetic approaches to determine their importance in the development and progression of lesions. Besides the unguided transcriptomic analyses, I’m focusing on NADPH oxidase (NOX2) given its prominent elevation in microglia/macrophages in MS lesions and because my initial analysis of RNA databases shows its upregulation. Finally, a collaboration with chemist Dr. Ling has generated a less hydrophobic analog of the NOX2 inhibitor. I will evaluate this new drug in MS models.
Researcher: Atefeh Rayatpour

Multiple sclerosis lesion showing cells (blue) and an extracellular matrix protein that inhibits remyelination (yellow)
Rianne Gorter
Overcoming barriers to remyelination: elucidating the extracellular matrix proteome in MS
In multiple sclerosis (MS), brain cells called oligodendrocytes, which make the insulating myelin sheaths around nerve fibers, are lost. Without this protection, nerve fibers gradually degenerate, leading to worsening disability over time. The brain does attempt to repair this damage by forming new myelin, a process called remyelination. Unfortunately, as MS advances, remyelination becomes less efficient. This is likely due to the presence of factors that inhibit repair, particularly extracellular matrix (ECM) molecules. My postdoctoral research aims to better understand which repair-inhibiting molecules are present in MS lesions and how they affect remyelination. Ultimately, this work seeks to inform the development of therapies that can overcome the inhibitory lesion environment and promote functional recovery.
Researcher: Rianne Gorter

Iron-induced lesion at day 3 stain for neuron, astrocyte and oxidized lipids. Cyan – NeuN (neuronal cell body), Yellow – GFAP (astrocytes), red (malondialdehyde)
Dorsa Moezzi
Iron-Induced cell death in the spinal cord: implications for neurodegenerative disease progression
Oxidative stress plays a critical role in neurodegenerative diseases, including multiple sclerosis (MS). Elevated iron levels have been observed in the basal ganglia of individuals with MS, even at early stages of the disease. Despite these observations, current treatments do not specifically address toxicity arising from iron accumulation. To investigate the effects of iron buildup in the central nervous system (CNS), we developed an in vivo model of iron toxicity and examined its contribution to oxidative stress and neuronal death. Neuronal loss was detectable as early as 2 hours post injection, with substantial loss evident by 1 day post injection. Motor coordination and balance deficits persisted in iron treated mice for up to 21 days. Notably, despite extensive tissue damage, the antioxidant defense system was not upregulated until 3 days post injury. These findings suggest that endogenous CNS defenses are insufficient to counteract iron induced oxidative stress, leading to neurodegeneration. Together, our results highlight the need for therapeutic strategies that directly target iron toxicity in diseases such as MS and provide a foundation for future studies on iron driven neurodegeneration.
Researcher: Dorsa Moezzi
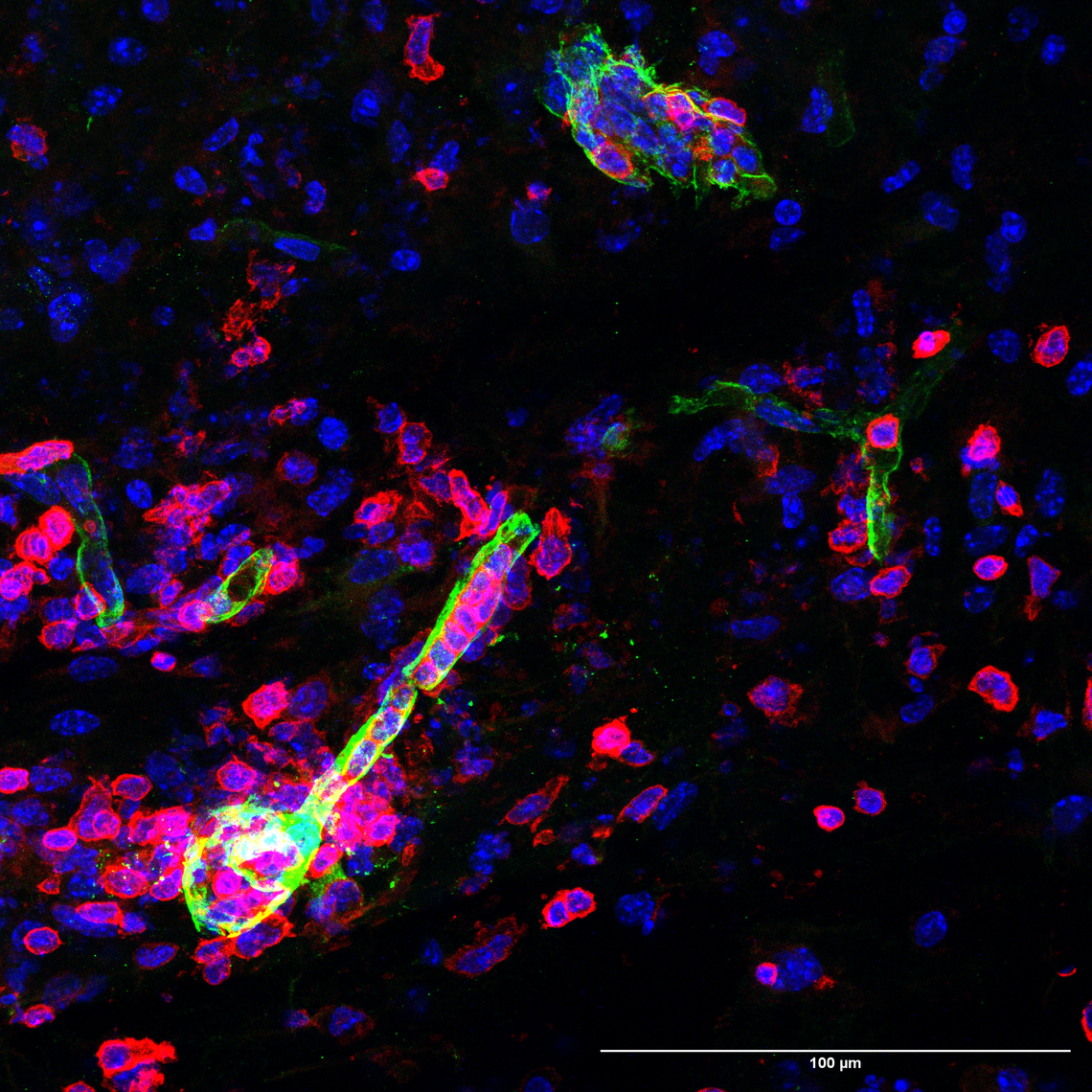
Immunofluorescence imaging of spinal cords from an animal model of MS. Barriers (green) are disrupted and immune cells (red) infiltrate the CNS parenchyma.
Maryam Mobarakabadi
Investigating roles of versican in the development and progression of EAE using transgenic mice
The extracellular matrix (ECM) surrounding cells is altered and dysregulated in CNS lesions of MS. Specific ECM molecules such as hyaluronan and chondroitin sulfate proteoglycans (CSPGs) accumulate in lesions, while others including tenascin-C and -R decline in active lesions. The ECM molecules and inflammatory cells can co-modulate each other. Moreover, some ECM molecules alter repair and remyelination processes. My project is focused on versican, a member of the lectican CSPG family, as it is prominently deposited in lesions of MS and its animal model, experimental autoimmune encephalomyelitis (EAE). Monocytes and macrophages appear to be the major sources of versican in EAE lesions5. Our lab has shown that versican enhances migration of monocytes through Matrigel and promotes the differentiation of T helper cells towards a cytotoxic Th17 phenotype. Both by directly affecting oligodendrocytes and by increasing Th17 toxicity toward oligodendrocytes, versican may inhibit remyelination processes. While previous research from our lab showed that inhibition of CSPG synthesis with difluorosamine ameliorates EAE severity, the drug inhibits all CSPG members and it is not selective to versican. Thus, the precise identification of versican as the detrimental CSPG member, and its cellular source and mechanisms, remain to be better defined. In my PhD project, I hypothesize that genetic deletion of versican from CCR2+ cells (mostly monocytes/macrophages) will prevent EAE development and progression.
Researcher: Maryam Mobarakabadi

Inflammation and neuronal damage in day 3 lysolecithin lesion in mouse spinal cord white matter. CD68 in red for microglia/macrophage. Neurofilament (NFH) in green for axons. Amyloid precursor protein in magenta. Captured at 25x on SP8.
Dennis Brown
Novel drug combination to attenuate neurodegeneration in an MS model
Current treatments for MS focus on relapse-remitting MS, in contrast to primary progressive MS (PPMS), which skips the relapse-remitting phase and disease immediately begins to progress. With a lack of current treatment options for PPMS, my research seeks to unveil an effective and affordable treatment for the PPMS population. We will investigate two inexpensive generic drugs: Indapamide, a high blood pressure medication and antioxidant, and Hydroxychloroquine (HCQ), an anti-malarial drug known to inhibit microglia, an immune cell of which increased activation is a hallmark of PPMS. Over-activation of microglia results in the degradation of myelin, an insulator surrounding nerve cells, along with myelin-producing cells. This breakdown releases molecules contributing to free radical formation, thus enhancing the risk of disease progression. Using in vitro models along with the lysolecithin mouse model of MS, we will observe the effects that the combination of these drugs has on neuron and oligodendrocyte protection, demyelination, and inflammation. With HCQ currently in a Phase 2 clinical trial for PPMS and indapamide demonstrating promising effects on free radicals in animals, there is optimism that in combination the drugs will have strong therapeutic potential, giving rise to a new treatment plan to fill the void that currently exists for PPMS.
Researcher: Dennis Brown

Isolated oligodendrocyte precursor cells from P1 mouse pups with Alexa 488 for O4 in green and DAPI staining for nuclei in blue. Imaged at 20x on ImageExpress.
Emily Jelinek
Versican V1 as a primary inhibitor of remyelination in multiple sclerosis
Following myelin destruction, the brain has an innate repair system in which immature cells called oligodendrocyte precursor cells (OPCs) migrate to the lesion, develop into mature cells, and replace the damaged myelin. There is an inhibitory environment in the MS lesion that prevents OPCs from entering and maturing into myelin-forming cells. The aim of my research is to overcome this inhibitory environment with a focus on chondroitin sulphate proteoglycans (CSPGs). I am studying a particular CSPG member in the CNS called versican V1. Versican V1 is of interest because while high during CNS development, it is not seen in the adult CNS until injury. My work involves tissue culture work and experiments with a transgenic mouse line. My hypothesis is that CSPGs, particularly versican V1, physically restrain process elongation, proliferation, migration and maturation of plated OPCs, and that the conditional removal of versican V1 in lesions will lead to enhanced OPC numbers and remyelination. There are no current therapeutic approaches to induce remyelination in MS and this study hopes to shed light on the most important inhibitor of OPCs and propose a specific targeting strategy that is permissive of remyelination.
Researcher: Emily Jelinek
