Fluorescence Lifetime Imaging Microscopy (FLIM) is based on the differences in exponential decay rates of fluorescent probes. Fluorescent probes' lifetimes, rather than intensity, are used for contrast. Each pixel contains a decay curve used to differentiate species. FLIM is sensitive to the local micro-environment. Unlike intensity-based contrast, it is more robust for quantitative imaging as it is independent of probe concentration and spectral signature, illumination intensity, and photobleaching. HBIAMP has two microscopes that utilise the TCSPC (Time-Correlated Single Photon Counting) method of detecting Fluorescence Lifetime (see Figure 1). For more details, see Leica FLIM and AMP Education page.

Figure 1: Jablonski diagram and Time Correlated Single Photon Counting methond of Fluorescence Lifetime Imaging
Applications
- Species separation
- Spectrally overlapping fluorescent probes separation
- Label-free endogenous autofluorescence contrast, eg tumor detection
- Autofluorescence removal
- Biosensing: detection of changes in micro-environment and metabolic activity
- Intracellular signaling & ion concentrations, pH, polarity, temperature, lipids
- Biochemical reactions & compositions
- Rapid kinetic studies at video-rate speeds.
- Molecular Interactions
- FLIM-FRET: molecular distances, protein interactions
- Molecular conformation and binding

Intensity- and lifetime-based image of Arabidopsis thaliana root–hypocotyl junction expressing an actin fluorescent marker and labeled with propidium iodide. Actin, cell wall, and chloroplasts show different characteristics in the FLIM image
Era, A. et al. Plant Cell Physiol. 50, (2009)
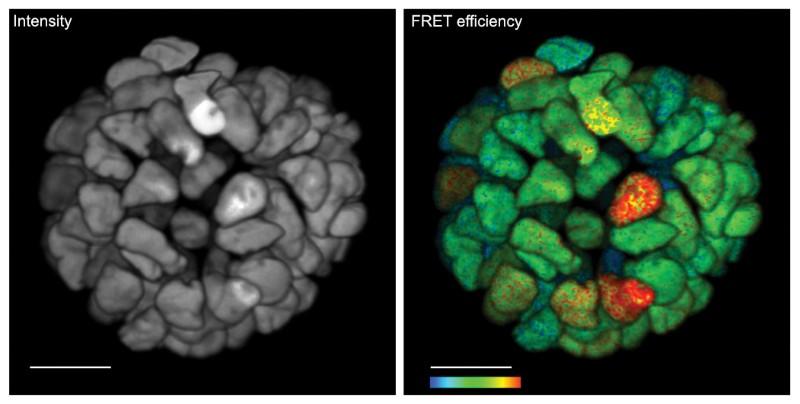
Live 3D FLIM on patient-derived organoid: Monitoring cancer-derived increased of signal transduction activity in live organoids with an ERK FRET-FLIM biosensor. Fluorescence intensity image (left) shows no functional information. FLIM image (right) shows FRET efficiency, ie ERK activity. Color bar, 2–12% FRET efficiency. Scale bar, 20 μm
van de Wetering, M. et al. Cell 161, 933–945 (2015)
Spectral Imaging combines microscopy and spectroscopy, mapping both intensity and emission spectra of fluorescent probes across a specimen. By using a multi-anode detector (Figure 2), each pixel contains intensity fluctuations as a function of spectral frequency (wavelength) which is unmixed to differentiate fluorescent probes based on their spectroscopic signature. Spectral imaging can be used to examine many fluorescent probes simultaneously ("multiplexing") and detect molecular interactions. For more details, visit Nikon MicroscopyU. Another method of spectral detection utilises a multi-band detection system (Figure 3) employing a glass prism for high transmission and white performance. The spectrum is separated into bands by means of movable mirror sliders. These allow the spectrum to be split in any possible set of sub-spectra which are then recorded as detection channels. When combined with a white-light laser, multiplexing of up to 11 colour channels are possible.

Figure 2: Light dispersion into its spectral frequencies (wavelengths) on a 32-channel multianode photomultiplier.

Figure 3: Multi-band detection system employing a glass prism for high transmission and white performance. The spectrum is separated into bands by means of movable mirror sliders. These allow the spectrum to be split in any possible set of sub-spectra which are then recorded as detection channels.
Spectral imaging is available on HBI AMP's Nikon C1si and Nikon A1R microscopes (links below). Equipped with 32-channel detectors, some of the systems capabilities are:
High-speed spectral imaging
Acquisition of 32-channel spectral images at standard confocal galvo-galvo speeds of up to 24 fps (512 x 32 pixels) or up to a high resolution of 2048 x 2048 pixels.
High-speed unmixing
Accurate spectral unmixing and real-time unmixing (A1R only) for the separation of overlapping fluorescence spectra and removal of autofluorescence.
Wide band spectral imaging
Simultaneous 4-laser excitation enabling spectral imaging across wider bands.
V-filtering function
Filter-less variable range detection to image optimally up to 4 fluorescent probes.
Applications
- Spectral unmixing of overlapping fluorescent probes and autofluorescence
- Quantitative Förster Resonance Energy Transfer (FRET)
- Spectral karyotyping
- Detection of changes introduced by drugs or pathological conditions
Fully tuneable, Prism-based Spectral Imaging is available on all our Leica Confocal Microscopes.

V-filtering function: Filter-less variable range detection of 3 probes by multianode photomultiplier binning

Spectral and 5-color unmixed images of HeLa cells
Dr. Tadashi Karashima, Department of Dermatology, Kurume University School of Medicine

